Articles by Laxmaiah Manchikanti, MD
and Mahendra Sanapati, MD

Intrathecal drug delivery systems, more commonly known as pain pumps, are typically used for management of chronic nonmalignant pain and cancer-related pain. However, these systems are also used in managing severe muscle spasticity. An intrathecal pain pump implant provides pain relief by sending pain medication through a thin, flexible tube in extremely small doses. Intrathecal drug delivery of pain medications and muscle relaxants allows for direct administration of spinally-administered agents to their location of action within the central nervous system.
Intrathecal drug delivery has several advantages for the treatment of chronic pain as compared to peripheral delivery. By administration through pain pump, medicine bypasses the first pass metabolism and bypasses the blood brain barrier. Due to the direct delivery of treatment drugs to the location of action, less drugs can be used, which can in turn result in less interaction with systemic receptors, leading to overall less adverse effects.
Who is a candidate?
An intrathecal pain pump implant may be used in chronic pain or cancer pain from an injury or a disease. It can also help ease pain when other types of pain care have not worked or have caused severe side effects. It may be used after trying pain medication by pill, liquid or injection. In addition, when all other treatments have failed and surgery is not an option, intrathecal pain pump is considered. For severe muscle spasms, a baclofen pump is considered.
Pump procedure
Intrathecal pain pump implantation requires 2 stages after selecting the patients with appropriate assessment and psychological evaluation.
TRIAL
Intrathecal pain pump trial is very similar to an epidural or spinal injection, wherein a small dose of pain medication, either Dilaudid short-acting or morphine long-acting is injected. Following the procedure, the patient is observed for at least 2 hours with activities and pain relief and functional improvement is judged.
PERMANENT IMPLANT
If appropriate relief of greater than 50% is obtained with improvement in functional status without side effects of urinary detention, nausea, vomiting, constipation, a person is considered to be a candidate for permanent implant.
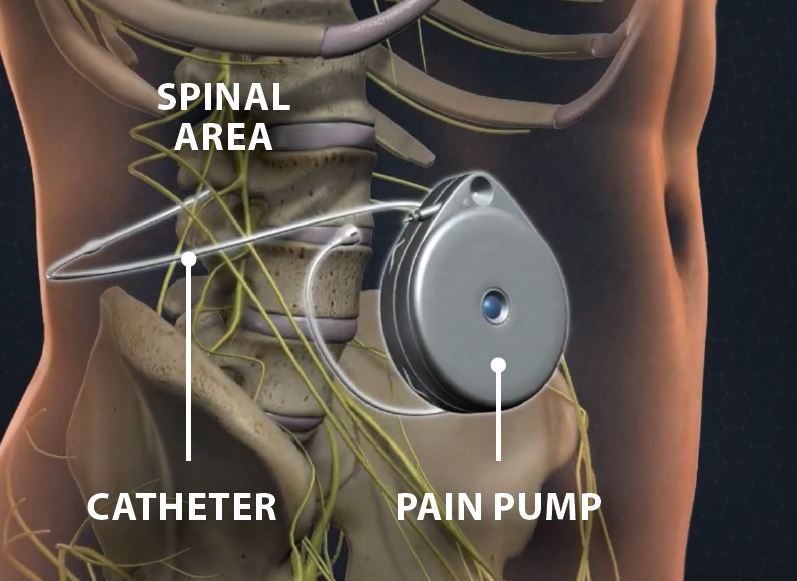
A permanent implant is placed by connecting a small tubing to a round pump. The tubing is placed from the lumbar spine into the mid back area. This is connected to a pump, which is placed in the lower back over the hip or in the abdominal area. A small electronic device controls the pump. The device stays outside the body. This is considered as a minimally invasive surgery. The pump contains medicine that sends it through the tube into the cerebrospinal fluid, which is circulating in the intrathecal space. The medicine reaches nerves along the spine. It helps prevent them from sending pain signals to the brain.
The type of medicine used in the pump will depend on your type of pain and other factors including allergies. The pump will need to be refilled with medicine when needed. This happens frequently in the majority of the patients in 2 months and rarely, every 1 or 3 months.
The intrathecal pain pump may be associated with risks including:
- Infection
- Small growth of tissue near the catheter called granuloma
- A tear in the catheter that stops medicine from getting to the nerves
- Errors in programming of the device
- Damage to the nerves of the spine
- Leaking of the cerebrospinal fluid that causes headache and other symptoms
- A pocket of cerebrospinal fluid under the skin called hygroma
- A pocket of the other fluid under the skin called seroma
- Other complications are related to pain medication and its usual side effects
What’s new?
In managing chronic pain conditions, some pain pumps have advanced. Currently, there are several pump systems in the market. The life of a pain pump is approximately 5 years, after which time the battery needs to be replaced.
